Which Best Describes the Difference Between Internal and Thermal Energy
It is the portion of potential energy that can be transferred from one substance to another. Which best describes thermal energy.

4 Laws Of Thermodynamics Science Guide Thermodynamics Physics Physics Formulas
Unit of internal ene.

. It is the sum of internal energies of two or more substances. It increases when the temperature of the body rises or when the body changes from solid to liquid or from liquid to gas. It is the portion of internal energy that can be transferred from one substance to another.
It is the difference between internal energies of two or more substances. It is not in transit but remains as part of the internal energy of the system. Which description best describes heat.
Which best describes thermal energy. It is the portion of internal energy that can be transferred from one substance to another. Thermal energy is the energy obtained by an object due to the motion of its particles.
When the kinetic energy of particles in a substance decreases what also decreases. Which best describes thermal energy. Heat on the other hand is energy in transit ie.
It is the portion of internal energy that can be transferred from one substance to another. Internal energy is the sum of kinetic and potential energy of all particles in the body. Internal energy is the energy stored in a body.
What follows is my understanding of the distinction between the two which I hope will be of use to all science teachers. Which best describes the relationship between heat internal energy and thermal energy. Which best describes thermal energy.
Thermal Energy and Internal Energy. April 14 2019 emc2andallthat. Therefore we can conclude that it is the portion of internal energy that can be transferred from one substance to another.
It is the portion of potential energy that can be transferred from one substance to another. It is the portion of potential energy that can be transferred from one substance to. How Does Heat Differ From Thermal EnergyThe difference between heat and thermal energy is that thermal energy is not in the process of being transferred.
It is the difference between internal energies of two or more substances It is the sum of internal energies of two or more substances O It is the portion of internal energy that can be transferred from one substance to another. So to answer the first question the difference between thermal energy and internal energy is that internal energy includes all energy except that which is due to external interactions whereas thermal energy only includes a subset of internal energy that is due to thermal excitations. It is the portion of potential energy that can be transferred from one.
The difference between heat and thermal energy is that thermal energy is not in the process of being transferred. Heat is the transfer of kinetic energy from one medium or object to another or from an energy source to a medium or object. Val Its particles have less kinetic energy.
Which statement describe the thermal energy of an object. It is not in transit but remains as part of. It is the sum of internal energies of two or more substances.
It is the difference between internal energies of two or more substances It is the sum of internal energies of two or more substances It is the portion of internal energy that can be transferred from one substance to another It is the portion of potential energy that can be transferred from one substance to another. It is the difference between internal energies of two or more substances. Answer 1 of 8.
It is the difference between internal energies of two or more substances. The thermal energy of an object is the energy contained in the motion and vibration of its moleculesThermal energy is measured. Thermal energy is heat that flows and heat is the part of internal energy that can be transferred.
Internal energy is the total potential and kinetic energies in a substance and thermal energy is the part of internal energy that can be transferred to another substance. It is the sum of internal energies of two or more substances. 2 on a question.
The AQA GCSE Science specification calls for students to understand and apply the concepts of not only thermal energy stores but also internal energy. It is also known as internal energy as it is the energy within the particles due to their motion. Which best describes thermal energy.

Tia 568 C Fiber Optic System Commercial
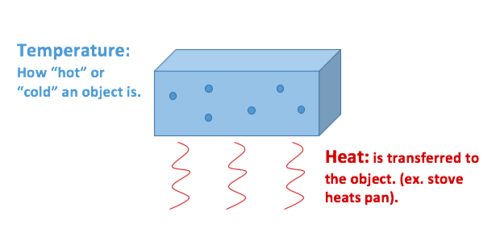
Heat Vs Temperature Energy Education

3 Way Switch Wiring 3 Way Switch Wiring Schematic Drawing Three Way Switch
No comments for "Which Best Describes the Difference Between Internal and Thermal Energy"
Post a Comment